How Is Orbital Hybridization Useful in Describing Molecules
Browse the archive of articles on Nature Chemistry. The structures of alkanes and other organic molecules may also be represented in a less detailed manner by condensed structural formulas or simply condensed formulas.
Parsing Spdf Orbital Hybridization And Simple Bonding
Ionic Hydrogen bonding dipole dipole Van der Waals.
. Vsepr lab answer key. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. Three representative fingerprints ie FP2 Hybridization and Daylight were selected to express the chemical structure of the 10 new molecules.
It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. In chemistry a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pairLone pairs are found in the outermost electron shell of atoms. The key thing to consider here is that boiling points reflect the strength of forces between molecules.
Instead of the usual format for. The nuclear skeleton of the Lewis Structure of these resonance structures remains the same only the. The more they stick together the more energy it will take to blast them into the atmosphere as gases.
Count the pi electrons. The first case has two pi electrons 2 in the pi bond zero in the p orbital of the carbocation which is a Huckel number. The second case has 4 pi electrons 2 in the pi bond 2 in the p-orbital of the carbon which bears the negative formal charge.
It is not aromatic. Vsepr lab answer key Animals. 4 is not a Huckel number.
There are 3 important trends to consider. The results are displayed in table S5. Soft bioelectronic devices have exciting potential applications in robotics computing and medicine but they are typically restricted by the.
The comparison between the prediction results by the RF model and the experimental PCE values is. A molecule or ion with such delocalized electrons is represented by several resonance structures. Academiaedu is a platform for academics to share research papers.
Vsepr lab answer key. The relative strength of the four intermolecular forces is. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
They can be identified by using a Lewis structureElectron pairs are therefore considered lone pairs if two electrons are paired but are. Because of the sp 3 hybridization the bond angles in carbon chains are close to 1095 giving such chains in an alkane a zigzag shape. Well-dispersed molecules on the sidewalls of CNTs outer diameters of 12 nm were observed in NiPc MDEsfree of observable aggregation of NiPcs Supplementary Fig.
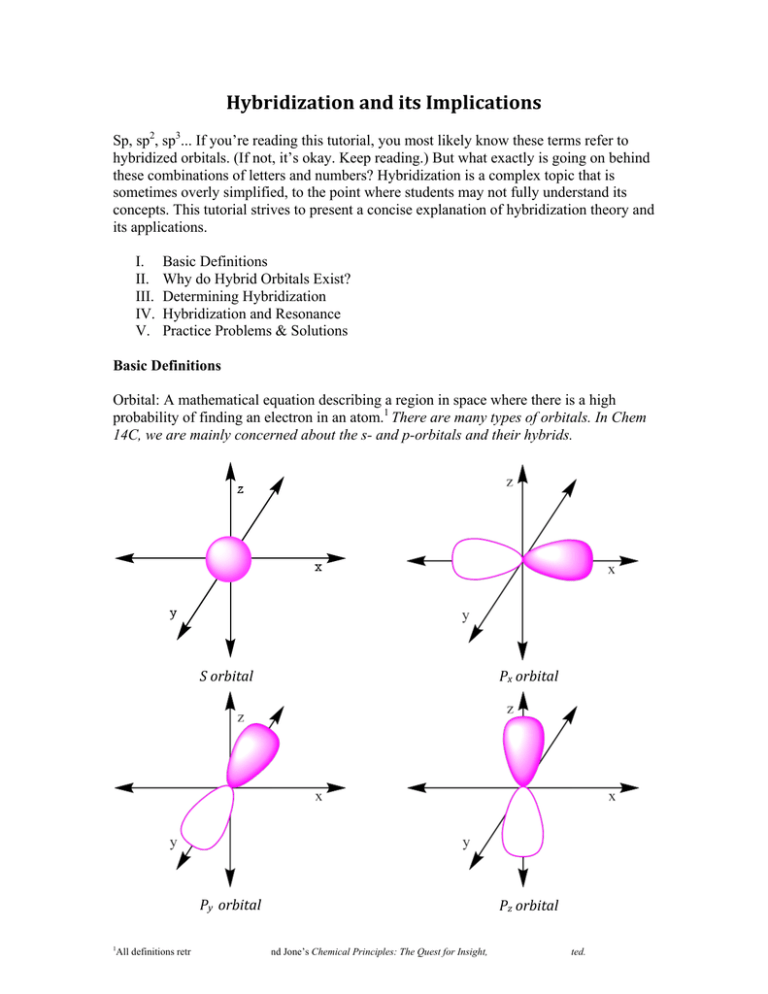
Hybridization And Its Implications

Molecular Orbitals 8 3 How Are Atomic And Molecular Orbitals Related Ppt Video Online Download

8 3 A Sigma Bond Molecular Orbitals Ppt Video Online Download
0 Response to "How Is Orbital Hybridization Useful in Describing Molecules"
Post a Comment